In recent talks at physics departments about my book, I have emphasized that the elementary “particles” of nature — electrons, photons, quarks and so on — are really little waves (or, to borrow a term that was suggested by Sir Arthur Eddington in the 1920s, “wavicles”.) But this notion inevitably generates confusion. That’s because of another wavy concept that arises in “quantum mechanics” —the quantum physics of the 1920s, taught to every physics student. That concept is Erwin Schrödinger’s famous “wave function”.
It’s natural to guess that wave functions and wavicles are roughly the same. In fact, however, they are generally unrelated.
Wavicles Versus Wave Functions
Before quantum physics came along, field theory was already used to predict the behavior of ordinary waves in ordinary settings. Field theory is useful for sound waves in air, seismic waves in rock, and waves on water.
Quantum field theory, the quantum physics that arose out of the 1940s and 1950s, adds something new: it tells us that waves in quantum fields are made from wavicles, the gentlest possible waves. A photon, for instance, is a wavicle of light — the dimmest possible flash of light.
By contrast, a wave function describes a system of objects operating according to quantum physics. Importantly, it’s not one wave function per object — it’s one wave function per system of interacting objects. That’s true whether the objects in the system are particles in motion, or something as simple as particles that cannot move, or something as complex as fields and their wavicles.
One of the points I like to make, to draw the distinction between these two types of waves in quantum physics, is this:
- Wavicles can hurt you.
- Wave functions cannot.
Daniel Whiteson, the well-known Large Hadron Collider physicist, podcaster and popular science writer, liked this phrasing so much that he quoted the second half on X/Twitter. Immediately there were protests. One person wrote “Everything that has ever hurt anyone was in truth a wave function.” Another posted a video of an unfortunate incident involving the collision between a baseball and a batter, and said: “the wave function of this baseball disagrees.”
It’s completely understandable why there’s widespread muddlement about this. We have two classes of waves floating around in quantum physics, and both of them are inherently confusing. My aim today is to make it clear why a wave function couldn’t hurt a fly, a cat, or even a particle.
The Basic Concepts
Wavicles, such as photons or electrons, are real objects. X-rays are a form of light, and are made of photons — wavicles of light. A strong beam of X-ray photons can hurt you. The photons travel across three-dimensional space carrying energy and momentum; they can strike your body, damage your DNA, and thereby cause you to develop cancer.
The wave function associated with the X-ray beam, however, is not an object. All it does is describe the beam and its possible futures. It tells us what the beam’s energy may be, but it doesn’t have any energy, and cannot inflict the beam’s energy on anything else. The wave function tells us where the beam may go, but itself goes nowhere. Though it describes a beam as it crosses ordinary three-dimensional space, the wave function does not itself exist in three-dimensional space.
In fact, if the X-ray beam is interacting with your body, then the X-ray beam cannot be said to have its own wave function. Instead, there is only one wave function — one that describes the beam of photons, your atoms, and the interactions between your atoms and the photons.
More generally, if a bunch of objects interact with each other,the multiple interacting objects form a single indivisible system, and a single wave function must describe it. The individual objects do not have separate wave functions.
Schrödinger’s Cat
This point is already illustrated by Schrödinger’s famous (albeit unrealistic) example of the cat in a box that is both dead and alive. The box contains a radioactive atom which will, via a quantum process, eventually “decay” [i.e. transform itself into a new type of atom, releasing a subatomic particle in the process], but may or may not have done so yet. If and when the atom does decay, it triggers the poisoning of the cat. The cat’s survival or demise thus depends on a quantum effect, and it becomes a party to a quantum phenomenon.
It would be a mistake to say that “the atom has a wave function” (or even worse, that “the atom is a wave function”) and that this wave function can kill the cat. To do so would miss Schrödinger’s point. Instead, the wave function includes the atom, the killing device, and the cat.
Initially, when the box is closed, the three are independent of one another, and so they have a relatively simple wave function which one may crudely sketch as
- Wave Function = (atom undecayed) x (device off) x (cat alive)
This wave function represents our certainty that the atom has not yet decayed, the murder weapon has not been triggered, and the cat is still alive.
But this initial wave function immediately begins evolving into a more complicated form, one which depends on two time-varying complex numbers C and D, with |C|2 + |D|2 = 1:
- Wave Function = C(t) x (atom undecayed) x (device off) x (cat alive) + D(t) x (atom decayed) x (device on) x (cat dead)
The wave function is now a sum of two “branches” which describe two distinct possibilities, and assigns them probabilities |C|2 and |D|2, the former gradually decreasing and the latter gradually increasing. [Note these two branches are added together in the wave function; its branches cannot be rearranged into wave functions for the atom, device and cat separately, nor can the two branches ever be separated from one another.]
In no sense has the wave function killed the cat; in one of its branches the cat is dead, but the other branch describes a live cat. And in no sense did the “wave function of the atom” or “of the device” kill the cat, because no such wave functions are well-defined in this interacting system.
A More Explicit Example
Let’s now look at an example, similar to the cat but more concrete, and easier to think about and draw.
Let’s take two particles [not wavicles] A and B. These particles travel only in a one-dimensional line, instead of in three-dimensional space.
Initially, particle B is roughly stationary and particle A comes flying toward it. There are two possible outcomes.
- There is a 30% probability that A passes right by B without affecting it, in which case B simply says “hi” as A goes by.
- There is a 70% probability that A strikes B head-on and bounces off of it, in which case B, recoiling from the blow, says “ow”.
In the second case, we may indeed say that A “hurts” B — at least in the sense of causing B to recoil suddenly.
The Classical Probabilities
Before we answer quantum questions, let’s first think about how one might describe this situation in a world without quantum physics. There are several ways of depicting what may happen.
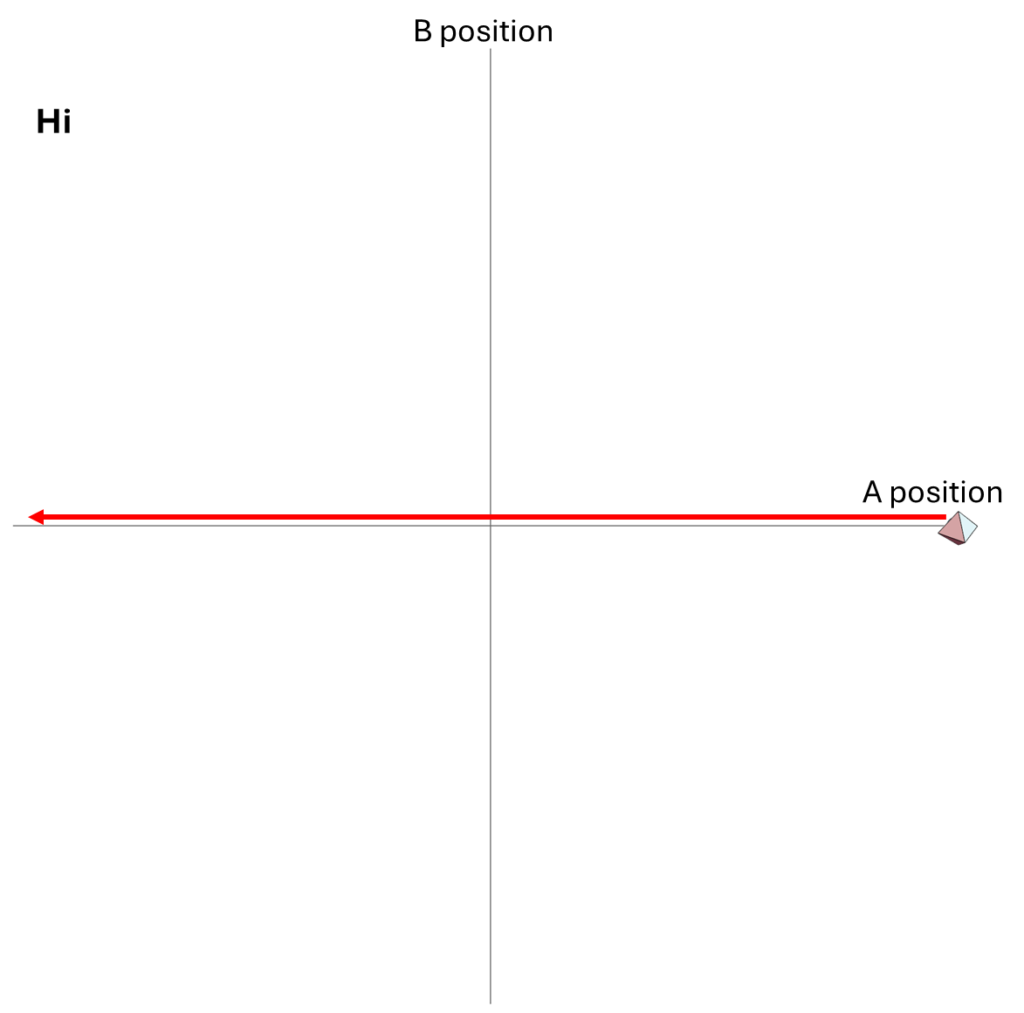
Motion in One-Dimensional Physical Space
We could describe how the particles move within their one-dimensional universe, using arrows to illustrate their motions over time. In the figure below, I show both the “hi” possibility and the “ow” possibility.
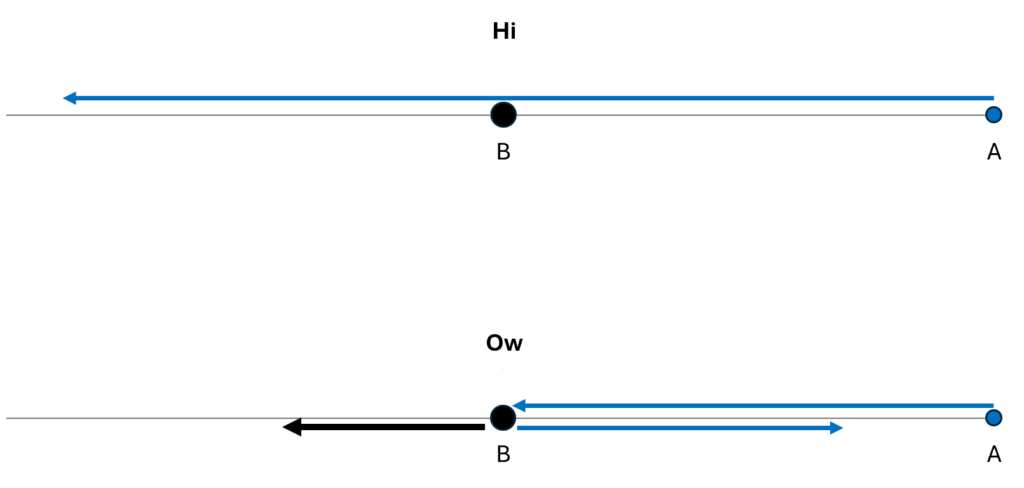
Or, using an animation, we can show the time-dependence more explicitly and more clearly. In the second case, I’ve assumed that B has more mass than A, so it recoils more slowly from the blow than does A.

Motion in the Two-Dimensional Space of Possibilities
But we could also describe how the particles move as a system in their two-dimensional space of possibilities. Each point in that space tells us both where A is and where B is; the point’s location along the horizontal axis gives A’s position, and its location along the vertical axis gives B’s position. At each moment, the system is at one point in that space; over time, as A and B change their positions, the location of the system in that two-dimensional space also changes.
The motion of the system for the Hi and Ow cases is shown in Fig. 3. It has exactly the same information as Fig. 1, though depicted differently and somewhat more precisely. Instead of following the two dots that correspond to the two particles as they move in one dimension, we now depict the whole system as a single diamond that tells us where both particles are located.
In the first part of Fig. 3, we see that B’s position is at the center of the space, and remains there, while A’s position goes from the far right to the far left; compare to Fig. 1. In the second part of Fig. 3, A and B collide at the center, following which A moves to positive position, B moves to negative position, and correspondingly, within its space of possibilities, the system as a whole moves down and to the right.
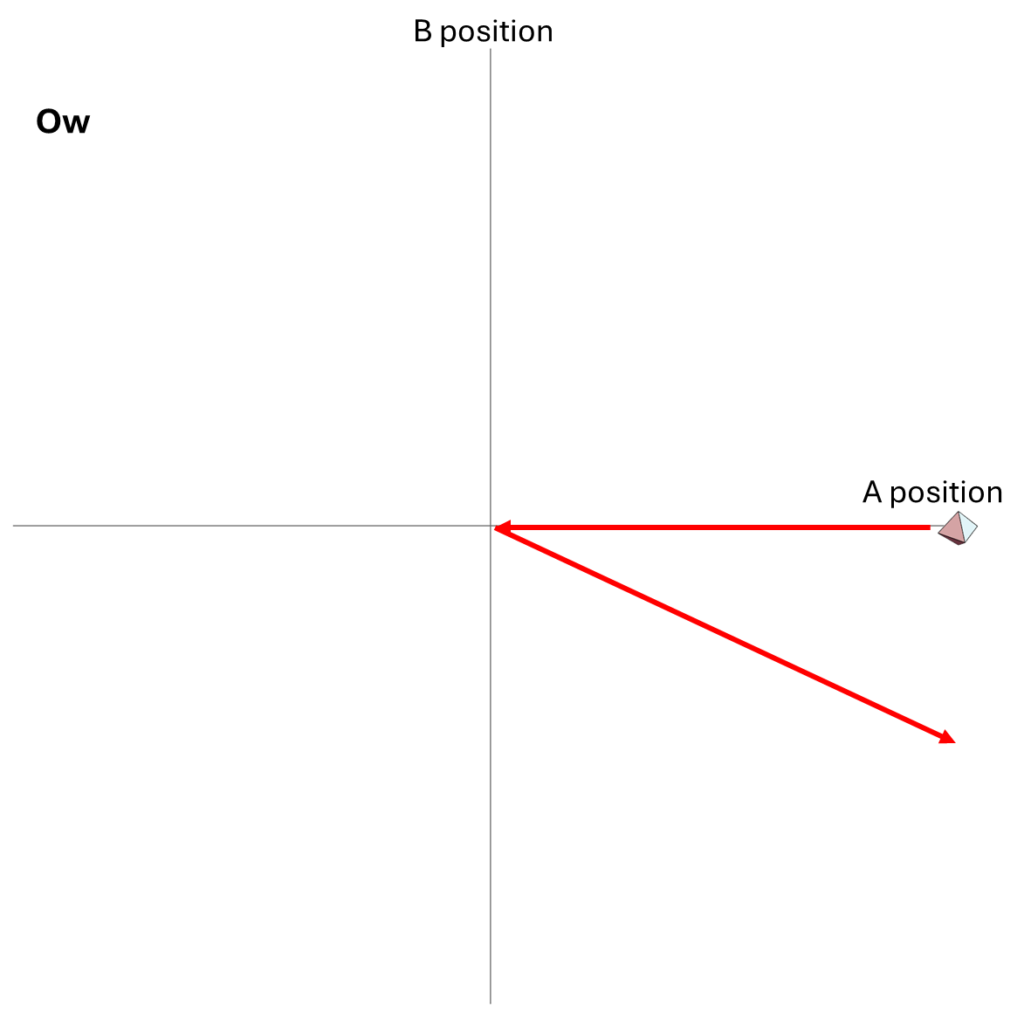
And finally, let’s look at an animation in the two-dimensional space of possibilities. Compare this to Fig. 3, and then to Fig. 2, noting that it has the same information.

In Fig. 4, we see that
- the system as a whole is represented as a single moving point in the space of possibilities
- each of the two futures for the system are represented as separate time-dependent paths across the space of possibilities
The Quantum System
Now, what if the system is described using quantum physics? What’s up with the system’s wave function?
As noted, we do not have a wave function for particle A and a separate wave function for particle B, and so we do not have a collision of two wave functions. Instead, we have a single wave function for the A/B system, one which describes the collision of the two particles and the aftermath thereof.
It is impossible to depict the wave function using the one-dimensional universe that the particles live in. The wave function itself only exists in the space of possibilities. So in quantum physics, there are no analogues to Figs. 1 and 2.
Meanwhile, although we can depict the wave function at any one moment in the two dimensional space, we cannot simply use arrows to depict how it changes over time. This is because we cannot view the “Hi” and “Ow” cases as distinct, and as something we can draw in two separate figures, as we did in Figs. 3 and 4. In quantum physics, we have to view both possibilities as described by the same wave function; they are not distinct outcomes.
The only option we have is to do an animation in the two-dimensional space of possibilities, somewhat similar to Fig. 4, but without separating the Hi and Ow outcomes. There’s just one wave function that shows both the “Hi” and “Ow” cases together. The square of this wave function, which gives the probabilities for the system’s possible futures, is sketched in Fig. 5.
[Note that what is shown is merely a sketch! It is not the true wave function, which requires a complete solution of Schrödinger’s wave equation. While the solution is well known, it is tricky to get all the details of the math exactly right, and I haven’t had the time. I’ll try to add the complete and correct solution at a later date.]
Compare Fig. 5 with Fig. 4, recalling that the probability of the Hi case is 30% and the probability of the Ow case is 70%. Both possibilities appear in the wave function, with the branch corresponding to the Ow case carrying larger weight than the branch corresponding to the Hi case.
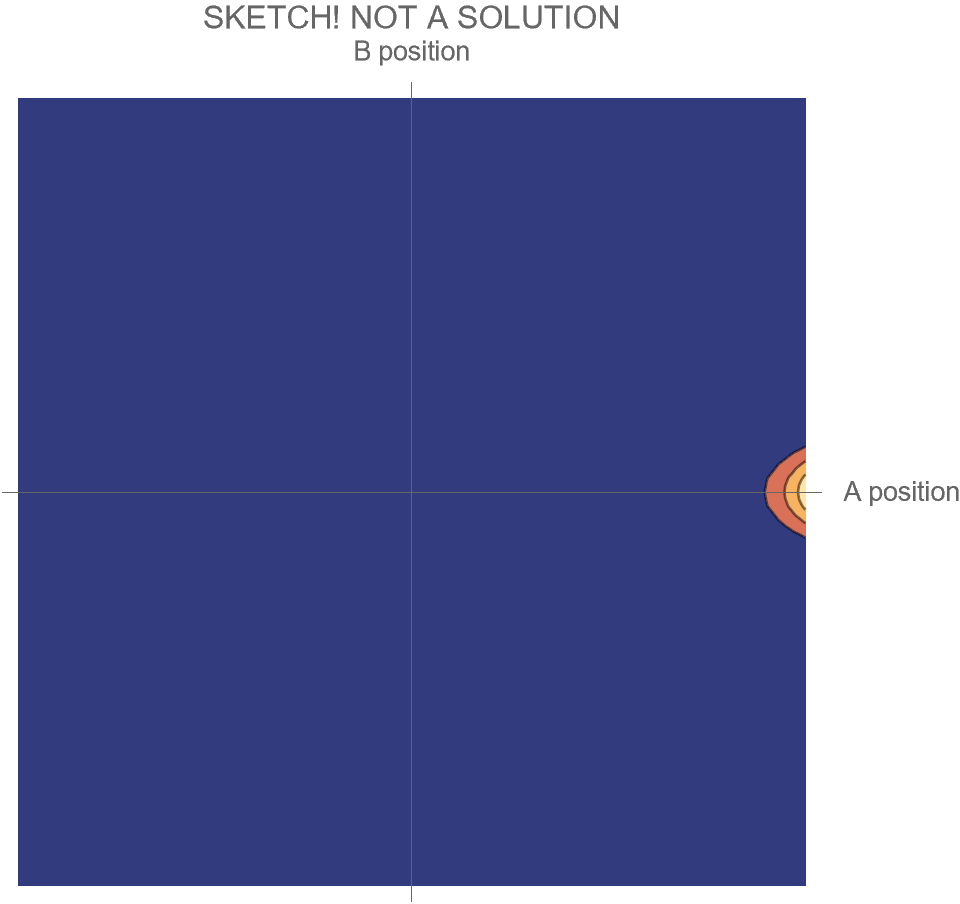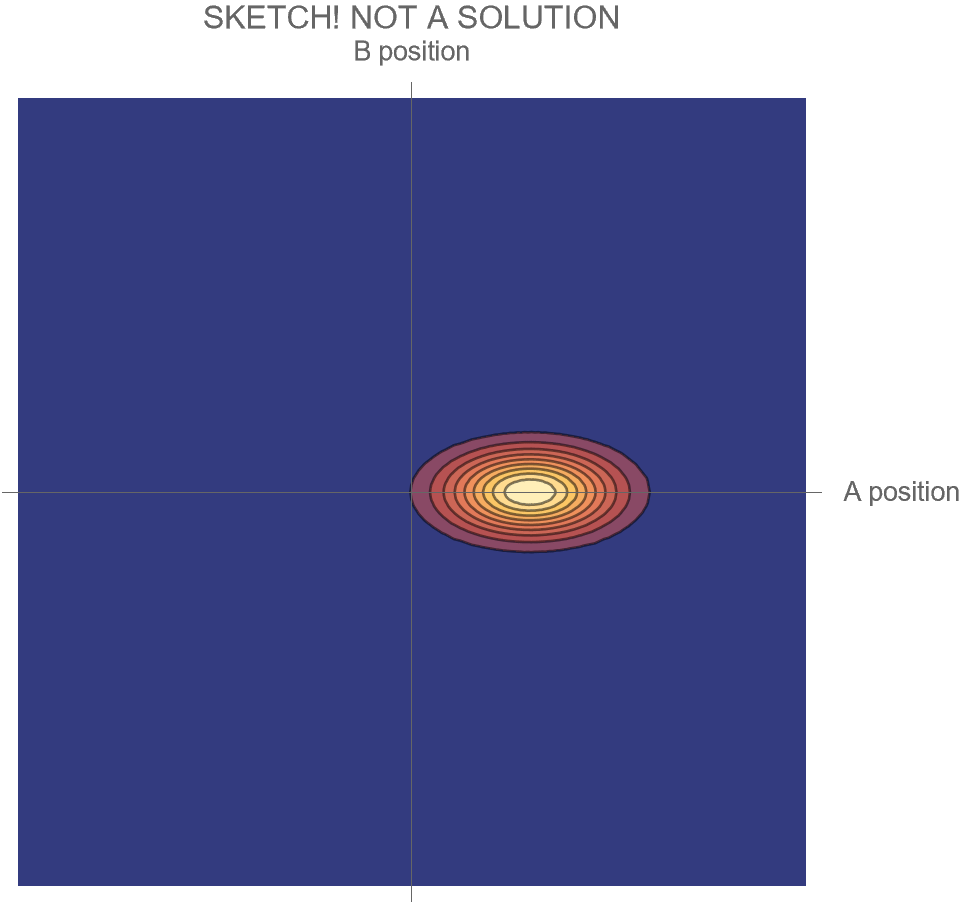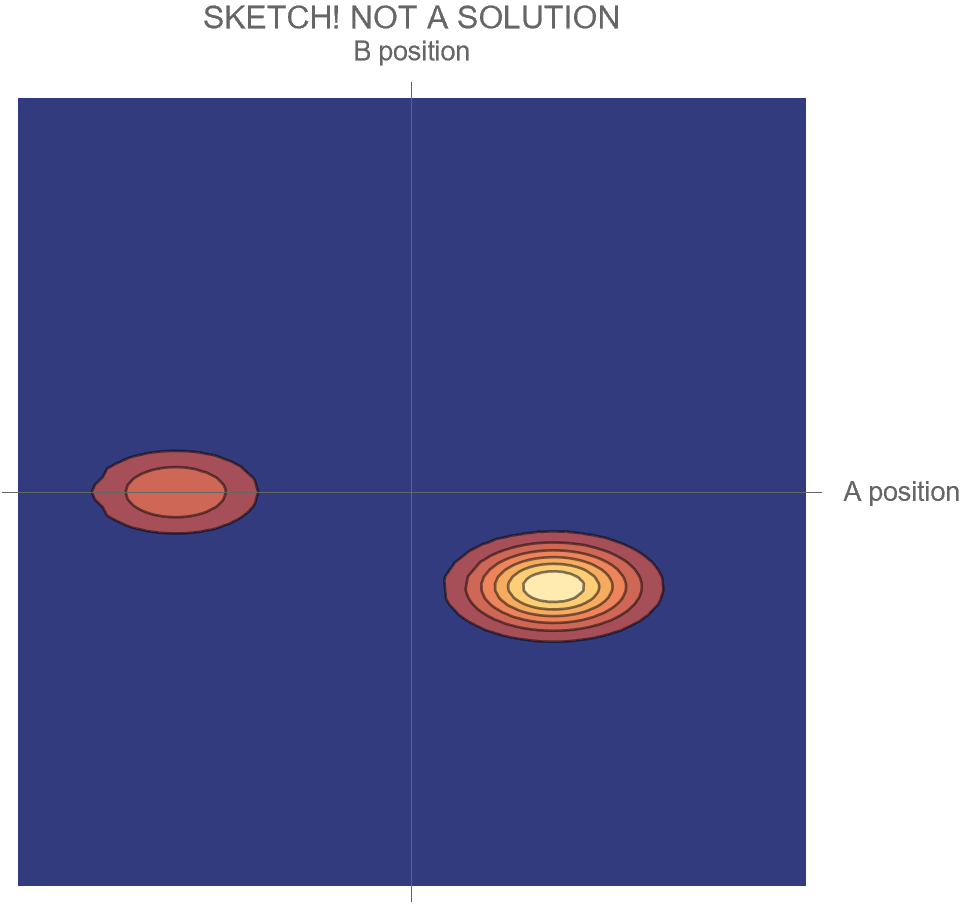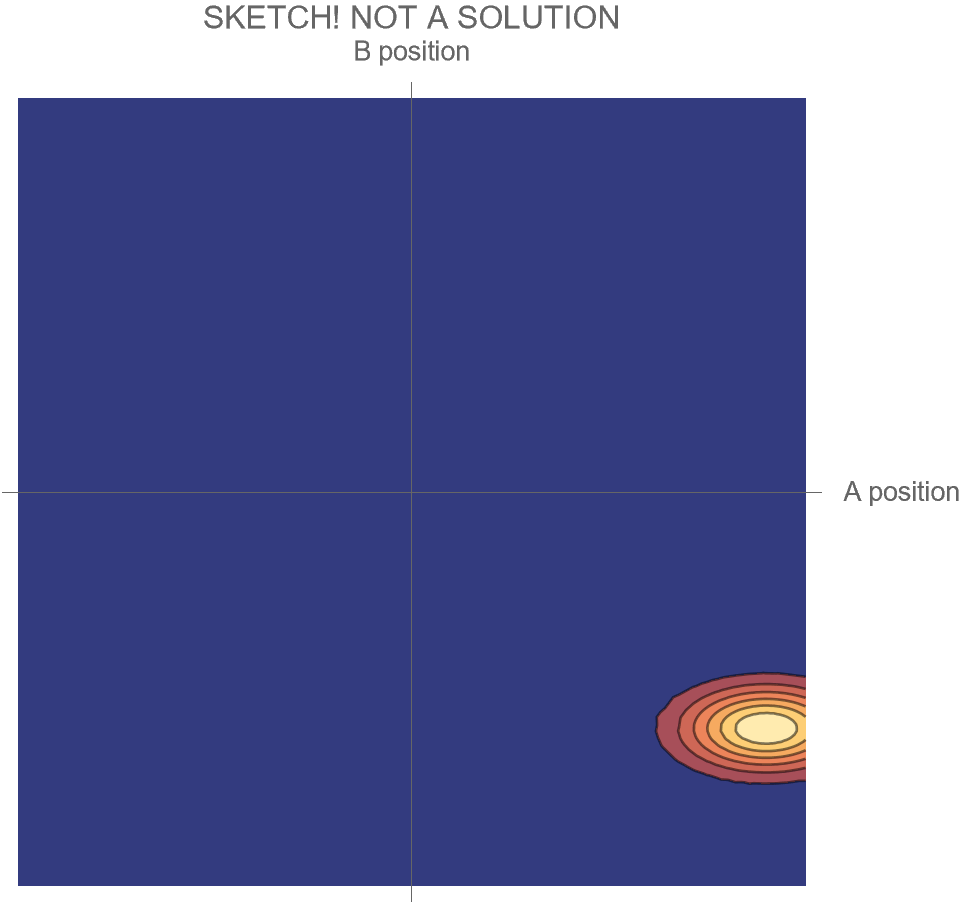
In contrast to Fig. 4, the key differences are that
- the system is no longer represented as a point in the space of possibilities, but instead as a (broadened) set of possibilities
- the wave function is complicated during the collision, and develops two distinct branches only after the collision
- all possible futures for the system exist within the same wave function
- this has the consequence that distinct future possibilities of the system could potentially affect each other at a later time — a concept which makes no sense in non-quantum physics
- the probabilities of those distinct futures are given by the relative sizes of the wave function within the two branches.
Notice that even though particle A has a 70% probability of “hurting” B, the wave function itself does not, and cannot, “hurt” B. It just describes what may happen; it contains both A and B, and describes both the possibility of Hi and Ow. The wave function isn’t a part of the A/B system, and doesn’t participate in its activities. Instead, it exists outside the system, as a means for understanding that system’s behavior.
Summing Up
A system has a wave function, but individual objects in the system do not have wave functions. That’s the key point.
To be fair, it is true that when objects or groups of objects in a system interact weakly enough, we may imagine the system’s full wave function as though it were a simple combination of wave functions for each object or group of objects. That is true of the initial Schrödinger cat wave function, which is a product of separate factors for the atom, device and cat, and is also true of the wave function in Fig. 5 before the collision of A and B. But once significant interactions occur, this is no longer the case, as we see in the later-stage Schrödinger cat wave function and in Fig. 5 after the collision.
A wave function expresses how the overall system moves through the full space of its possibilities, and grows ever more complex when there are many possible paths for a system to take. This is completely unrelated to wavicles, which are objects that move through physical space and create physical phenomena, forming parts of a system that itself is described by a wave function.
A Final Note on Wave Functions
As a final comment: I’ve given this simple example because it’s one of the very few that one can draw start to finish.
Wave functions of systems with just one particle are misleading, because they make it easy to imagine that there is one wave function per particle. But with more than one particle, the only wave functions that can easily be depicted are those of two particles moving in one dimension, such as the one I have given you. Such examples offer a unique opportunity to clarify what a wave function is and isn’t, and it’s therefore crucial to appreciate them.
Any wave function more complicated than this becomes impossible to draw. Here are some things to consider.
- I have only drawn the square of the wave function in Fig. 5. The full wave function is a complex function (i.e. a complex number at each point in the space of possibilities), and the contour plot I have used in Fig. 5 could only be used to draw its real part, its imaginary part, or its square. Thus even in this simple situation with a two-dimensional space of possibilities, the full wave function cannot easily be represented.
- If we had four particles moving in one dimension instead of two, with positions x1, x2, x3 and x4 respectively, then the wave function would be a function of the four-dimensional space of possibilities, with coordinates x1, x2, x3, x4 . [The square of the wave function at each point in that space tells us the probability that particle 1 is at position x1, particle 2 is at position x2, and similarly for 3 and 4.] A function in four dimensions can be handled using math, but is impossible to draw.
- If we had two particles moving in three dimensions, the first with position x1, y1, z1, and the second with position x2, y2, z2, the space of possibilities would be six-dimensional — x1, y1, z1, x2, y2, z2 . Again, this cannot be drawn.
These difficulties explain why one almost never sees a proper discussion of wave functions of complicated systems, and why wave functions of fields are almost never described and are never depicted.





50 Responses
Thanks for the post — I hadn’t come across the wavicle terminology before, but I have had the distinction in my head for a while. I think someone below asked something similar, but I’d be very happy if you could clarify a couple of points.
If I had a scalar field $\phi(x)$, what would you call the function $\braket{ k | \phi(x) | 0}$ (where $\ket{k}$ is a momentum eigenstate and $\ket{0}$ is the vacuum)?
Similarly, if I thought of a particle moving between two spacetime points $x_1$ and $x_2$, what would you call the Feynman/time-ordered propagator $\Delta_F{x_1 – x_2}:= \braket{0 | T(\phi(x_1) \phi(x_2)) | 0}$?
Both are complex-valued and correspond to amplitudes for some process. And I believe the first is often called the single-particle wavefunction (perhaps erroneously, and not just because of what you say above). And I think that when taking the non-relativistic limit to calculate e.g. the anomalous magnetic moment of the electron, or the Lamb shift in hydrogen, we effectively treat $\braket{k | \psi_e(x) | 0}$ as an electron wavefunction.
Now suppose I had an imaginary universe populated only by $\phi(x)$ (and no gravity), which now has self-interactions and (for whatever reason) is not at in its vacuum state. Would it be right in your view to say that the only real wavefunction is the *wave-functional* of the field $\Psi[\phi(x)]$?
Sorry, there are some details missing. Are your first questions about a free theory or an interacting theory, and if the latter, are there any other fields? Does the particle associated to \phi have mass? And is the ket{k} a one-particle momentum eigenstate or a general momentum eigenstate of arbitrary particle number? From your last question, I think you want the first questions to be about a free theory of phi, and the last one to be about a self-interacting theory of phi, but I want to be sure.
> Are your first questions about a free theory or an interacting theory, and if the latter, are there any other fields? Does the particle associated to \phi have mass? And is the ket{k} a one-particle momentum eigenstate or a general momentum eigenstate of arbitrary particle number?
For the first two questions, let’s start by taking $\phi$ to have a mass $m$ and be free, and let $\ket{k}$ be a one-particle (free) momentum eigenstate for simplicity, and let’s assume no other fields. I’m curious if your interpretation would change if e.g. we added a $\lambda \phi^4$ term here — I agree that the vacuum state would change and particle number would no longer be conserved but I wouldn’t think it would affect the “wavicle”/”wavefunction distinction”.
> From your last question, I think you want the first questions to be about a free theory of phi, and the last one to be about a self-interacting theory of phi
Yes, I wanted to relax the assumption of a free theory because I thought that would make the vacuum state (maybe I am prejudicing the discussion with this language) too uninteresting as we wouldn’t have virtual particles and the field’s wavefunctional would correspond to some state of definite particle number.
Nothing changes in wavicle vs wavefunction when you add interactions, assuming the theory still actually has “particle” states with mass, i.e. assuming there is an eigenstate of the Hamiltonian with finite energy corresponding to a single, isolated particle. In this case, there can be any number of wavicles. But there is only one wave function.
That said, many other important things do change. And you risk getting many things wrong if you don’t use caution in relying on free QFT to gain intuition about interacting QFT. The two theories are infinitely far apart. Your understanding of an interacting QFT needs to be non-perturbative, even if you then go ahead and use perturbation theory to do certain calculations.
For instance, in your last paragraph, when you say “virtual particles” and “definite particle number”, you have potentially used the word “particle” in two completely different ways. There are two propagators appearing in interacting QFT: the full two-point function (phi phi) , whose pole represents the physical particle with mass M, and the free two-pointfunction i/(p^2-m^2) , in which the mass m is just a parameter. Virtual particles involve the free propagator, and only exist when doing perturbation theory. What exactly do you mean by “definite particle number”? Definite numbers of free particles? Or definite numbers of physical particles? A non-perturbative definition of particle-number counting has to do with how many particles of mass M actually show up in my detector. But I don’t think that’s always what you meant.
More generally, focusing on phi(x)|0> is not a good idea for defining one particle states. You should focus on the Hamiltonian and its eigenstates, which include the physical one-particle states. Since phi(x)|0> has indefinite energy, it creates indefinite numbers of physical wavicles in the interacting theory. Said another way, it’s the energy spectrum of the interacting theory that tells you what a physical particle is, not the free Fock space.
For these reasons, I don’t want to answer your original questions, not because they don’t have answers but because some of them are based on assumptions that are true in free QFT but not in an interacting QFT. Ask only questions that make sense in both free and interacting QFT, and I’ll be happier to answer those.
Now, back to the original issue. Wavicles are things that can be absorbed in detectors and counted. There is only one wave function Psi[phi], no matter what state the field is in. The wave function of the field can only be written as a product of wave functions of individual wavicles [or rather, a symmetrized product to account for Bose statistics] if those wavicles are non-interacting, which requires either that the QFT is free or that the wavicles are in a state such that they are always far apart. In the latter case, they must be in localized and always-far-separated wave packets; they cannot be in plane waves of definite momentum since they would overlap somewhere. If any of the particles interact with one another, then the wave function of the field cannot be factorized. Only in free QFT can the wave function Psi[phi] always be written as a Bose-symmetrized product of one-particle wave functions.
Note also that if there are massless particles also (as in the case of electrons and photons) then there are additional complexities since there are photons of arbitrarily low energy that can be added to a one-electron state… or equivalently, the electron has a long-range electric field to which arbitrarily low energy can be added even if the electron is at rest.
To reiterate: focus on interacting QFTs and understand them, and use free-QFT intuition only when it’s very clear that it will not be misleading.
> There is only one wave function Psi[phi], no matter what state the field is in … If any of the particles interact with one another, then the wave function of the field cannot be factorized. Only in free QFT can the wave function Psi[phi] always be written as a Bose-symmetrized product of one-particle wave functions.
Thanks, this matches what I’ve known and clarifies what the wavicle/wave-function distinction is. And from your penultimate comment I take it you’re referring to difficulties with “soft” photons, this time in specifying the wave-function rather than in terms of an infrared divergence.
> Said another way, it’s the energy spectrum of the interacting theory that tells you what a physical particle is, not the free Fock space.
I agree with this, and to put it another way: poles of the exact propagator correspond to physical one-particle states, or as you’ve now clarified, “wavicles”.
> More generally, focusing on phi(x)|0> is not a good idea for defining one particle states
Yes, but one often sees $\phi(x)\ket{0}$ (or $\braket{k| \phi|0}$ ) in introductory QFT books being used to define a wavefunction of sorts e.g. Peskin & Schroder or Schwartz. I just wanted to check if wavicle idea would resolve this misuse of terminology.
I appreciate your detailed response and the caveats you’ve raised in order to avoid saying something that is technically incorrect, or using concepts that are ill-defined, but I think you are being far too restrictive in specifying how one should formulate a question!
> Ask only questions that make sense in both free and interacting QFT, and I’ll be happier to answer those.
To be clear, in terms of particle number etc. I was talking about the free particle number operator and indeed defining everything with respect to the free fields. If you’re really being strict about it, it’s not clear that there is a rigorous notion of an interacting QFT at all — so shouldn’t we stick to defining things in terms of free operators?
> To reiterate: focus on interacting QFTs and understand them, and use free-QFT intuition only when it’s very clear that it will not be misleading.
This intrigues me somewhat, because apart from a few very special cases, how many interacting QFTs are exactly soluble?
Put it another way, the first two courses one takes in QFT are generally taught in the context of perturbation theory — at least as far as I’m aware. The traditional treatments of scattering and the S-matrix involve assuming the existence of free field operators (which satisfy the free-field equation of motion with the physical mass $m_P$) and basis states in the asymptotic future/past, and it’s with respect to these in and out states that we calculate scattering cross-sections and rates.
Yes there is mention of the Kallen-Lehmann exact propagator, and when deriving the LSZ rule we hear that the external “legs” of the proper vertices are dressed with $\frac{iZ}{p^2 – m_P^2}$, where $m_P$ is the physical mass and $Z$ is the “wavefunction” renormalization factor, you don’t really approach nonperturbative physics until you get non-Abelian gauge theory/QCD, in which case it’s all treated semi-classically anyway.
(By the way, would a soliton count as a wavicle?)
To avoid the wrong impression: I absolutely agree with you that it is a bad habit to think of QFT as purely an exercise in perturbing around the simple solutions — despite the formal training one receives at university. It’s much, much richer than that. It’s simply that in the context of /scattering/ — which is what I think we’re talking about when we talk about detecting particles (and I might be mistaken!) — I find it hard to have any sense of an “exact” treatment.
To me, the notion of free field is intimately connected with the classical notion of particle (or wavicle), precisely because the Hamiltonian of a free field is what one naively expects a theory of particles to be: simply the sum of all the energies of the particles. Once we add interactions, we lose that direct connection — but instead we see that since the pole in the free field propagator corresponds to a particle, in the interacting case we take the poles in the exact propagator to be one-particle states.
Keeping the full interacting theory in mind would be hard as it’s not even clear what the particle states are in those cases. For instance, in Yang-Mills theory we can write down the usual Lagrangian but how on Earth can we tell from that alone what the particle states look like? Yes, it turns out that thinking gluons are the physical degrees of freedom at large scales is very wrong, but at least it’s true at small distances.
And a final point is that the notion of what counts as a particle is also observer dependent, even for free fields. I did take care to avoid gravity in the final question, but perhaps you would also want me to specify that there were no accelerating observers around as well, carrying their own particle detector while maintaining their own notion of the vacuum? 🙂
The fact that most courses and books on QFT are highly misleading at many levels is indisputable. I teach the subject quite differently, but life is short and there’s only so much time to write books or lecture notes. My treatment of SUSY QFT at TASI 2001 and 2003 gives some idea of how I approach it. I would put a lot of focus on interacting QED, on SUSY, on d less than 4, and on large Nc where the intuition for interacting QFTs is far better than for non-SUSY free QFT.
I’m not sure what you mean about there not being a rigorous notion of interacting QFTs. We have many in 1+1 dimensions, d just below 4, in Banks-Zaks theories, and in lattice models in d above 2; and it is clear that many interacting supersymmetric CFTs and QFTs exist even though we don’t have a precise definition that would satisfy a mathematician. Even though we do not have many exactly soluble interacting QFTs, we have many partially soluble ones; and we know what others do, from data and from simulation. Both particle physics and condensed matter physics give us a clear understanding of a wide variety of interacting QFTs, in which it is clear how the spectrum works, how operators work, and how n-point correlation functions work. It’s all very different from free QFT.
As for solitons and whether they are wavicles — by the time we are advanced enough to deal with quantized solitons, the word “wavicle” is losing some of its value, and the same is true of “soliton”. The spectrum of many supersymmetric theories contain multiple particle states, some of which carry electric charge, some of which carry magnetic charge, and some of which carry both; and which is which depends on what variables you choose to write the theory. In some regions of parameter space, where there is a weakly-interacting description, it may be possible to write some of these states as quantized solitons and to write others as nearly-free wavicles; but across the full parameter space, and in specific regions where there is no weakly-coupled description, there is no such choice, and so the question doesn’t have a useful answer.
The observer dependence is yet another wrinkle. Again, the only useful way to think about this correctly is to first understand the full non-perturbative content of the theory… for example, consider how wrong one’s understanding of accelerated observers in Yang-Mills theory would be without an understanding of confinement and deconfinement.
Hi Matt. Thanks for your explanation. I understand now that normally a particle described by quantum field theory consists of a superposition of various energy states. Also, of course, a wavefunction in quantum mechanics can be expressed as a sum of the energy wavefunctions, with the coefficients giving the probability that the particle has that particular energy. Does the superposition of energy states given by quantum field theory also assign a probability to each energy state, and if so do these probabilities agree with the probabilities given by the wavefunction? Or is is the case that it is only the wavefunction that can tell you the probability of a particle having each possible energy, and the superposition in quantum field theory just tells you that the particle has a mixture of energies, but does not tell you the probability of it having each energy in the mixture.
Kind regards,
Julian Collins
If you’re just looking at one isolated particle, for instance a particle in a box, then quantum field theory and quantum mechanics give you all the same information. A particle can be in a superposition of energy states in both quantum mechanics and quantum field theory. There’s a wave function in quantum field theory too, and in this case it is simply a translation of the one-particle wave function that you have in quantum mechanics. All the superposition and the probabilities are exactly the same.
Everything interesting in quantum field theory has to do with the number of particles being changeable and ambiguous, and the effects of relativistic interactions among particles. These issues cannot be treated consistently in quantum mechanics. A wave function in field theory that is not limited to a single, non-interacting particle is therefore vastly more complex than what one sees in quantum mechanics, and so quantum mechanics misleads by making things seem way too simple. Unfortunately, drawing, describing or even writing mathematics for wave functions in quantum field theory is simply too difficult, and other methods have to be used. But basic notions of superposition and probabilities still apply — they just apply to a dramatically more complicated system than that of a fixed number of particles interacting via a potential energy function.
Hi Matt. In quantum mechanics as described by the Schrodinger equation, an electron, for example, can be in a mixed state of energies, where it doesn’t have a definite energy but has a certain probability of having certain different energies. Do the equations of quantum electrodynamics give rise to a similar situation, where an electron has no definite energy, or does an electron always have a definite energy in QED. If it always has a definite energy in QED, how is this consistent with the probabilistic energy of the Schrodinger equation?
In quantum field theory, just as in quantum mechanics, most states of the theory are superpositions of states with different energy, with states of definite energy [energy eigenstates] being the exception. Any quantum theory allows superposition — it’s part of the structure of quantum physics — and so this is automatic.
What is really new in field theory is the possibility of superpositions of states with different numbers of particles. [The definition of “particle” is also subtle.]
Actually, though, even this is an overstatement about what is new. If you use quantum mechanics to make a model of a metal crystal, with atoms forming a stable grid, you will discover, in the solution, that the quantized sound waves of the crystal (“phonons”) obey the rules of quantum field theory. You will discover that superpositions of states with different numbers of phonons are allowed by the solution. Thus, in a sense, the rules of quantum field theory are already implicit in ordinary quantum mechanics.
Hi! I was having a very similar question, so glad you answered it in a post here. I did have a few more questions, sorry if these have been asked elsewhere.
First, what is the precise relation between a wavicle and a quantum field’s creation operator? Does the operator just create a wavicle?
Second, one common theme in both the book and in this post is the reality of wavicles, especially over wave functions. But aren’t wavicles just as unreal in some instances as wave functions? I ask because a quantum field can exist in a superposition of having different numbers of particles in it, each possibility weighted by a probability. That means it’s possible for a quantum field to exist in a superposition of both having and not having a wavicle- implying this wavicle is itself in a superposition of both existing and not existing at the same time! This seems just as unreal to me as a wave function.
Lastly, let’s say we have a quantum field that we know for sure has a single particle- we can express this as the field being in a superposition of having a particle with differing momenta. This is because one can write creation operators in terms of operators that add a particle with some momentum to the field. One can then weight each possible field in this situation by its probability of occurring and add them all together. This seems very similar to taking the plane wave representing a definite momentum state in classical QM, weighting it by the probability the particle has that momentum, and then adding it all up to get the wavefunction of the particle. Could this be a way to relate wavicles and wave functions too?
1) Yes. In free QFT, anyway; in interacting QFT, physical wavicles aren’t quite so simple.
3) This is how a wavicle in QFT is related to a *one-particle* wave function in QM. But it doesn’t work beyond one particle. Take two particles. Even in QFT, you have to tell me how the two wavicles are correlated, i.e. in momentum space, Psi(k1, k2) |k1,k2>. You can then get the two particle wave function from Psi, but there are still two wavicles described by that single wave function.
2) I don’t want get into a discussion of “what is real?” because that’s neither obvious nor obviously useful. I agree that “a quantum field can exist in a superposition of having different numbers of particles in it, each possibility weighted by a probability.” and “That means it’s possible for a quantum field to exist in a superposition of both having and not having a wavicle-“, but I disagree that this implies “this wavicle is itself in a superposition of both existing and not existing at the same time”. The world is what is in a superposition, not the wavicle. As you say, the world may or may not have a wavicle in it, and in the former case, the wavicle is a real object that could hurt someone who comes into contact with it. This is true of all objects in our universe; they may or may not exist, depending on which branch of the universe’s wave function your are looking at; but if they exist, they can hurt other objects that exist in that same branch. The wave function sits above them all, and hurts no one.
What’s the purpose behind using a diamond to represent a point in the classical picture, rather than just a point?
If you were to combine the classical ‘hi’ and ‘ow’ animation as you did for the quantum one, would I be correct in saying that the pointers after a potential collision would carry a different colour to one another to emphasize that their combined probabilities are one?
I didn’t want to use a dot or anything else ball-like, because I didn’t want people to see any resemblance between the particles A and B that make up the system and the symbol I used to represent the A/B system as a whole. I considered an X or a square but couldn’t get it to look nice enough.
You could combine the hi and ow animations in many ways, but it would be potentially confusing. In the quantum case, we have to combine them, so it is necessarily confusing.
I found your text very clear in explaining what you’re doing, and whereas the first animation has two balls representing the physical collision, there’s a single diamond in the second animation forcing us to think carefully about what this diagram means compared to the first. Hence replacing the diamond with a yellow ball would add to its clarity IMO: I was thinking there was some significance to the red/blue colouring and orientation of the triangles making up the diamond.
In fig 4 for ‘hi’, I believe the incoming diamond should change colour after passing the origin to reflect that it’s taken the 30% probability branch; and similarly another colour for the 70% probability ‘ow’ branch. To keep this consistent with the quantum picture later on.
Overall, I find it very confusing, and unintentionally misleading, to treat the classical and quantum cases as being drastically different to one another when in fact, they elegantly continuously merge into one another just by looking at your fig 5 quantum animation: as the interaction become more classical the two wave functions on each branch become less fuzzy and more localized. But the total probability for each branch remains invariant at 30% and 70% respectively.
So, it sounds like the idea is that there is only a single wave function for the entire universe, but it can be approximated by a collection of multiple such wave functions for individual subparts, particles, etc. So when interaction or measurement occurs, in truth, it is something going on within that single wave function. But of course the Schrödinger equation doesn’t model such an interaction. This makes it sound like only part of the wave function “collapses”. I have never heard anyone ever speak of a “partial collapse” though. Obviously the measurement problem is unsolved and there are various other interpretations, etc.
Though, it’s still unclear to me what the wavicles are doing when the wave function is diffusing out. Is the idea that the wavicle actually occupies two places at once? The wave function simply models the probabilities and not the actual physical wavicle object, which is thought of as the single unified object but actually physically diffused across space?
The Schrödinger equation most definitely does model that interaction. A measurement is an interaction, and the Schrodinger equation that corresponds to the Standard Model’s quantum field theory will indeed include it. [You can see the interaction between A and B in my example as a measurement of A’s momentum; it’s carried out only 70% of the time, but when it works, the reaction of B reflects the motion of A.] The equation even predicts that your belief about the outcome of a measurement will agree with what the particle in the measurement actually did.
What quantum mechanics doesn’t explain is why the wave function offers so many, many possibilities for the world. But it explains why what we believe happened in an experiment is correlated with what actually happened. (This is why the Many-Worlds interpretation of quantum physics is consistent, whether or not you subscribe to it.)
The wave function tells you the possibilities and the corresponding probabilities for those possibilities. In each of those possibilities, the wavicles do what they do; they do many bizarre things, but they don’t do logically contradictory things, such as being in a place and not being in a place at the same time.
Your question “Is the idea that the wavicle actually occupies two places at once?” is misleading in two directions. First, a wavicle can be two places at once simply because it’s a wave — a ripple from a rock thrown into a pond is many places at once, after all. But what you are really asking is whether the fact that the wave function has many branches implies that all of them are real at the same time. Well, that’s kind of the question, isn’t it? What does “real” mean?
All I can tell you is what we know: the wave function does not predict what will happen in the universe. It predicts certain sets of correlations between things that happen — an undecayed atom correlated with a living cat, a decayed atom correlated with a dead cat. It does not literally say the cat is both alive and dead. It says only that you do not yet know whether the cat is alive or dead, and either possibility may be true. When you open the box, the wave function does not collapse; it still has two branches. One has an undecayed atom correlated with a living cat correlated with your realization that the cat is alive; the other has a decayed atom correlated with a dead cat correlated with your realization that the cat is dead. The wave function (and Schrodinger’s equation for it) are just fine, but they do not pick the branch that you will experience. Perhaps they are unable to because quantum physics is incomplete, as Einstein believed. Perhaps they are unable because all branches are simultaneously real (and in each one, you will have a unique experience, if you exist there.) That’s the Many-World interpretation. Perhaps there are other options.
To see what is truly surprising about the wave function and quantum physics — the really freaky things about quantum physics that are impossible in non-quantum probability — we need to go much deeper. I’ll try to do this eventually, but this is a real morass, very difficult pedagogically and conceptually, and I am not ready. I avoided this challenge in my book for a reason!!
Have you read and studied Everett’s original paper?
“Relative State” Formulation of Quantum Mechanics
https://journals.aps.org/rmp/abstract/10.1103/RevModPhys.29.454
Sydney Coleman described this paper as ‘wonderful’ in his answer to a question at the end of his talk: Quantum Mechanics in your Face. So I’m wary when people make the claim that the Copenhagen and Many-Worlds interpretations are equivalent to one another. There seems to be far more going on in Everett’s analysis, such as questioning the foundations of probability, information etc, that isn’t appreciated by some theoretical physicists.
Well, they’re not equivalent; I think the Copenhagen interpretation isn’t consistent. It requires something for which there is no equation [i.e. wave-function collapse, which is clearly, in my mind, not a physical process but a metaphysical one — a change of the assumptions made about the system being described.]
The problem is that the Many-Worlds interpretation might not be right either. At least it is consistent with the equations.
Have you come across Harvard’s Jacob Barandes’s ideas on quantum mechanics?
He claims that: “Quantum theory can be reformulated in terms of old-fashioned configuration spaces and ‘indivisible’ stochastic laws, without a fundamental role for Hilbert spaces, wave functions, density matrices, or even the complex numbers.”
Yes, Jacob is a good friend of mine. He has interesting ideas and my opinion about them isn’t fixed yet.
But notice that he is saying “reformulated”, not replaced. Newton’s laws were rewritten multiple times, without any use of forces — i.e., without any F=ma equation or any notion of equal and opposite forces. His laws were reexpressed in terms of action, and then in terms of energy, and then even in terms of waves (https://en.wikipedia.org/wiki/Hamilton%E2%80%93Jacobi_equation). Reformulations aren’t breakthroughs, but they are often a good thing. They don’t actually change anything in the predictions, but sometimes they give you a new way to think about how you get those predictions. Jacob seems perhaps to have found a reformulation, but I don’t completely understand it yet, and so I’m not yet in a position to say how useful it may be.
I would say that you haven’t made the point as simply as you might: The wave function is a description of the system, not the actual stuff of the system. It’s like saying the number 10 can harm you if you drop a 10 pound rock on your head. Separately, the very term “wave function” is unfortunate because there’s nothing wave-like about it, other than in the simplest textbook fixed momentum single eigenmode case. It’s a terrible name, imo, that promotes the confusion you’re addressing.
Well, I don’t really agree that it’s like saying the number 10 can harm you if you drop a 10 pound rock on your head. The wave function describes you and and the rock and the injury. That’s far more subtle and complex than “10”.
Similarly, I don’t agreee with the statement that there’s nothing “wave-like about it, other than in the simplest textbook fixed momentum single eigenmode case.” First, a “fixed momentum single eigenmode case” is a sine wave, but physicists do not assign the word “wave” only to “sine wave”; any linear combination of sine waves, and thus all possible shapes, are all waves, for a physicist. [Also true for recording engineers and composers of electronic music, who speak of square waves and triangular waves and all sorts of others.] But the thing that really makes it a wave is the equation that it satisfies: local in time and space, and with wavelike solutions to which Fourier analysis can be applied. Specifically, if the wave function describes objects that have center-of-mass positions and move in physical space, then the “wave function” will satisfy an honest-to-goodness wave equation, with time dependence i d/dt and dependence on the center-of-mass positions that is a standard Laplacian. That defines a wave for me, and certainly did for Schrodinger as well; after all, his wave equation was immediately used to solve the problem of Hydrogen, in which the solutions are most definitely not sine waves, but everyone agreed, terminologically, that they were waves: solutions of a wave equation.
I’ll push back a bit and then leave it be: The point I was making is that the wave function and the Schroedinger equation describe the state of a physical system and how it will evolve. But the wave function is not the physical system itself any more than the position and momentum values of particles in a classical system are the particles themselves. It’s analogous to distinguishing a geometrical point from its coordinate system-dependent description. The coordinates, the wave function, the position and momentum values of classical objects: All are numerical attributes of the physical object that facilitate calculation, not the object itself.
Re. the wavelike nature of the wave function: I understand and agree that there’s a “wave equation” and you can solve it with Fourier series. But for me that doesn’t necessarily make it a wave (and I’m an ex-physicist too :D). But that’s just vocabulary, so I’m content to believe that your usage is more widely accepted. I do think it’s misleading to the lay audience, though, because people read “wave” and they think of something propagating through time and space. And while a wavefunction can do that, it’s also the case that a wave function describes my 10 pound rock sitting on the ground :D. Classical mechanics emerges as the h -> 0 limit of quantum mechanics, right? So what’s wavelike about the wavefunction describing the rock? It’s doesn’t spread or move in space to any appreciable extent, even though you can represent it as a linear combination of many fourier modes. That’s why I’m not a fan of the term “wavefunction”. But your mileage may vary…
On the first point, we basically agree.
On your second point: I agree that “wave function” is misleading, but for a different reason. It’s actually fine for rocks. You have perhaps forgotten that in relativity the rocks’ materials are all given by standing- or near-standing waves (even its center of mass.) In non-relativistic quantum mechanics, the rock’s wave function is hardly wavelike, but in relativistic quantum field theory it has an enormously high frequency f = mc^2/h, given by that very same wave function — it does indeed vary in time and in space according to a wave equation. (As discussed here and here… and central in my book’s thesis.)
I think a better example of where it is misleading is in systems that don’t exist in space, such as a set of spins, or more generally for any discrete quantum numbers that have nothing to do with motion in space. And a wave function for a quantum field satisfies an equation with derivatives with respect to the field’s values, not one with derivatives with respect to physical space. (These points might be worthy of a blog post…)
But as for changing the name… that’s a battle I wouldn’t win even if I tried. The problem is that wave functions of objects moving in physical space *are* wavelike [in the sense I used the term “wave”] and so that intuition is actually too useful to abandon. I’m trying to change “particle” to “wavicle”, but there I can argue that “particle” is almost always misleading; it almost always gives the wrong intuition.
Anyway, points taken.
And, even though I promised to leave it be, the simple reason “why a wave function can’t hurt you” is that the wave function is a description of the object not the object, itself. Only real point I was making… That’s why I thought the “10” of the 10 pound rock was a simple and fun way of making my point 😀
Done now, I promise (again).
Damn. I apologize for not having read your post more thoroughly. You actually made my point very explicitly. You wrote “The wave function associated with the X-ray beam, however, is not an object. All it does is describe the beam and its possible futures.” Sorry for the wind up and time sink.
No worries! Glad you did find that sufficient, in the end. In any case, your points about the weakness of the word “wave function” have been worthy of some thought.
Dr.Strassler:
I took physics many years ago, in college, and it was mostly classical physics, with an “introduction” to modern physics and quantum physics. I remember the first time I heard “wave nature” I immediately pictured a non-localized periodic wave, which was confusing. I then learned more about “wave packets” and Fourier series to construct the wave packet, which made more sense realistically, and localized the particle more. After reading your book, and subscribing to your blog, your description as “wavicles” has given the most clear picture of particles for me. The question: how would your wavicles be described relative to a wave packet? Are they equivocally the same? Is a wavicle a more modern notion of a wave packet?
A wavicle can take many shapes. A sine wave (i.e. a non-localized periodic wave) is one. A wave packet is another. But those are just two infinite classes out of an infinite set of options.
Wavicles are not restricted in shape, any more than a disturbance in the air is restricted to particular shapes. At any one moment, a wavicle can take any shape you want; it won’t stay that way, typically, since its “wave equation” will cause it to evolve over time.
The idea that one should use wave packets to make photons or electrons act more like particles is misleading. Indeed the word “particle” is misleading. Wavicles don’t have to act like them at all, except in one way (see below.) After all, electrons in atoms do not act like particles; the electron in a hydrogen atom surrounds the nucleus, which is not something that “particles” can do. Some electrons in a long organic molecule or in the conduction band of a metal can be spread out across many atoms, again an impossibility for a “particle”. Trying to make wavicles act like particles — and making wave packets out of them to make ourselves feel that we understand what makes them particle-like — is deeply misleading.
What makes a photon or an electron particle-like is only this: it is indivisible, has a definite rest mass, and can only be absorbed or emitted one at a time. Its shape is beside the point: it may be wave-packety for a certain amount of time, or it may be completely delocalized, or it may be heading in multiple directions at the same time, like a ripple in a pond. Trying to force a wavicle into a conceptual “particle”-box is not a good idea. Indeed, it’s exactly what I’m trying to move people away from.
Dr.Strassler:
I guess I should have been more explicit when I say “localized”. I don’t imagine a wavicle as being point like, I was viewing it similar to your explanation of standing wave, without walls, as it is not a point like object, it is spread out, just not spread out across the entire universe.
The issue is that you’re still trying to imagine that a wavicle has a definite shape. But wavicles can have any shape they like. They can be in a sine wave of any wavelength; and those simple waves can be combined (via Fourier analysis) into any shape you like — for a moment. That shape will then evolve. This, in fact, is true of any wave in any field. What makes the wave a wavicle is that its amplitude (not its shape) is constrained, though in a non-trivial way that requires a little too much math for me to lay out tonight. This is the same constraint that assures that if the wavicle is in a sine wave that has a frequency f, then its energy is E = f [h], where h is Planck’s constant.
Could you expand a bit on the concept of electrons taking up a variety of shapes? I’m thinking here in terms of atomic structure, where the electrons are assigned to various orbitals which have different shapes and energies: s orbitals are spherical, p orbitals are dumbbell-shaped, and d and f orbitals have yet more complex shapes. In addition, these orbitals can “hybridize” in various ways to accommodate different types of chemical bonding; for example, carbon (with a total of 6 electrons – 2 inert “inner” electrons and 4 “outer” electrons that participate in chemical reactions – can morph into tetrahedral sp3 orbitals for single-bonded molecules like methane (CH4), trigonal sp2 orbitals for double bonds (ethylene, CH2CH2), and linear sp orbitals for triple bonds (acetylene, CHCH). Throughout my career, these orbitals have been referred to as “probability regions” where an electron might exist, and chemistry has been explained on the basis of the occupancy of various orbitals. In other words, the orbitals have been treated as being separate and distinct from the electrons. Are you now saying that the orbitals actually ARE the electrons themselves? This may not change the underlying math that gives the orbitals their shapes, but it’s a huge difference in interpretation! And it does make sense when you think of the electrons as wavicles, but nonetheless it makes my head hurt a bit to visualize molecules this way!
I’m going to give you a short answer, not because you deserve one but because my best answer would be long, meandering, and still incomplete — and therefore probably not a very good one. When I have time, I plan to construct a better and more complete answer to this very obvious question.
Quantum field theory is vastly more complex than quantum “mechanics”, which in turn has to be simplified further, under various assumptions, to give the standard methods of quantum chemistry. So following how the field theory language descends to quantum chemistry language isn’t trivial; you have to make the right assumptions and watch how the math simplifies. In some cases I have done this, but I have not done it in enough settings to be sure I have it 100% right in all cases, or to explain it reliably to anyone else at this point.
It’s certainly not true that there will always a simple map between wavicle shapes in quantum field theory and orbital shapes in quantum chemistry. The wavicles still have a wave function which can encode all sorts of correlations that cannot be represented simply in space.
So I think the correct answer to your question is: “sometimes”. Such an identification works especially when the correlations among electrons are weak (other than those required by Fermi statistics) and all motions are slow relative to the cosmic speed limit; then the orbitals in quantum chemistry coincide in some way with the shapes of (or aspects of the shapes of) wavicles in quantum field theory… though the frequencies of the wavicles are vastly higher than quantum chemistry would suggest, since the latter ignores the mc^2 energy of the wavicles. (The fact that an electron wavicle’s shape is related to probabilities for measurements of its position, assuming the measurements are at distances much larger than the Compton wavelength of the electron, has to do with the spatial-locality of such measurements.) But this need not be the case in all circumstances. Hybridization is (I think) an example where correlations in the field theory wave function prevent you from making a simple identification. I’m sure things break down in atoms with relativistic inner shells. And I’m sure I don’t have the complete list of issues.
I’ll conclude by again warning you that I don’t have enough cases straight to be sure that this is the best answer.
Perhaps a useful way to think about this is to accept that the quantum chemistry orbital picture (both for isolated atoms and, as derived from molecular orbital theory, for molecules as well) is something of a cartoon version of whatever the “real” picture (from quantum field theory) may actually be. Nonetheless, even if it’s a only simplified “cartoon” version, the conventional orbital picture has a tremendous amount of explanatory and predictive power; as a case in point, the Woodward – Hoffman orbital symmetry rules of the 1960s even allow one to predict whether certain organic reactions will occur as thermal or photochemical processes, based simply on the algebraic signs of the pi orbitals of the molecules in question. It would therefore seem that the simple orbital picture is trying to tell us something about the underlying physical reality of atoms and molecules. It would be very interesting if a quantum field theory analysis of some relatively simple reaction that has already been studied in detail by quantum chemistry methods could be (or perhaps already has been) undertaken, to see what differences or similarities the two methods would reveal.
Quantum field theory differs from quantum mechanics only when special relativity is important. Is that ever true in standard chemistry? I know of no examples, but I’m no chemist. Perhaps issues arise in certain astronomical applications?
I am less clear about where molecular orbital theory and other methods of quantum chemistry differ from those of quantum mechanics. But I would expect them to fail mainly for atoms with many electrons, where the orbitals become very complicated — the electrons become highly correlated — and do not resemble those of the excited states of hydrogen. I could also imagine particular that there might be chemical reactions in which subtle details of wave functions, involving the interactions among electrons, are extremely important.
Even so, such cases would require quantum mechanics, and not quantum field theory. There’s no reason to expect wavicles to be needed for the standard chemistry of atoms in the upper half or so of the periodic table.
Basically, chemistry is a low-energy phenomenon, in the electron-volt range, while special relativity matters in the million electron-volt range. This is why the wavicle picture is not (or at least not often) important in chemistry, but is absolutely essential in understanding nuclear and particle physics, and perhaps even in extreme features of atomic physics (such as inner shell electrons in atoms at large atomic number.)
This helps to put things into perspective for me. Chemistry is certainly a low-energy phenomenon, and the orbital description of atoms and molecules has served very well over the years. I think I got confused by your comment in an earlier reply to another reader in which you implied that an electron in a long organic molecule could itself be spread out over a number of atoms. In the orbital picture of that molecule, we’d say that the orbital was delocalized over a number of atoms, and the electron (whatever size and shape it had) was free to roam around within that orbital — not that the electron itself was smeared out over all the atoms. But nonetheless, I think I’m comfortable with thinking of the electrons as wavicles that can occupy orbitals, if that makes any sense (pictorially, if not mathematically). Clearly, this is an involved topic!
Very good, this is a nice example. The orbital being delocalized on a long molecule certainly does mean the electron is delocalized.
This becomes clear when one looks at electrons in crystals, which is a nice, simple limit of this same idea. What I’m about to say applies to any system that is periodic in space.
In the quantum field theory of materials such as metals, the electrons are wavicles in periodic waves; the Fermi sea is built from them. This is also reflected in the quantum mechanics of metals, as was shown by Bloch: (https://en.wikipedia.org/wiki/Bloch%27s_theorem). While these Bloch waves can be understood as the hybridization of localized electron orbitals, the electrons really are extended waves in this example. Superconductivity would not work if this were not true.
For the same reasons, sound wavicles in the metal, namely “phonons”, are also extended waves. That might seem less surprising, but the point is that the math for the phonons and the math for the electrons is not that different.
(It is worth keeping in mind that all electrons are identical, and so it is not possible to say which electron is where once the electrons are overlapping.)
So this shows you why the fact that electrons are wavicles cannot be entirely forgotten, even in quantum chemistry, once you are looking at large systems where their wavelike properties dominate their behavior.
An astrophysical application where not special but general relativity issues arise is in cosmological redshifts. The sleight of hand explanation is that the photon “wavepacket” stretches to lower frequencies as it travels through expanding space.
Perhaps the conceptual touch point for the behavior may be that a wavicle assuming all shapes may sometimes be considered as a sine wave, in which case we expect the redshift and the changed energy?
So beautiful article, prof. Strassler. The two particles’ system, as an actually wave function, made me thinking about the Bayes theorem, as seen randomly on a Twitter math account to solve a quadratic function this morning: P(a/b)= P(b/a)P(a) divided by P(b). Is it possible that the 2 percentals of probabilities be not 70% and 30%? Finally, one of the two particle’ s behaviors depends on…what? Velocity, direction, nature of particles (electron, photon, etc.), nature of the system itself..? Thanks a lot.
I don’t personally know of any useful connection between wave functions and Bayes’ theorem, I’m afraid.
I am not sure I understand your final questions, but maybe this is the answer: I did not specify exactly how the balls interact, and what causes them to miss each other 30% of the time and collide 70% of the time. I could have done so, in which case the behaviors would depend on the strength (and perhaps other details) of the interaction between A and B, the speed of particle A, and the rest masses of both A and B. But the overall behavior of the wave function doesn’t depend on such details. For that reason, I chose not to be specific, so as not to clutter the (already long) discussion. Does that answer your question?
Not quite, but I trust I will learn a lot when I’ll read your new book. Thank you very much.
Great article! Thank you for the explanation.
Firstly, I agree with your basic premise regarding wavefunctions versus wavicles. Your description here raises long-time questions I have regarding wavefunctions of cats and large systems and whether a wavefunction for a cat-sized object is reasonable. As you point out in your Final Note, such a wavefunction would have a Hilbert space with a vast number of dimensions. As I understand it, it would need to contain all the information for each particle (plus all the particles of the system that either releases or doesn’t release the sleeping gas — I prefer to think of the cat as either sleeping or awake).
I guess my question involves the tractability or usefulness of such a wavefunction. The deeper question, perhaps, involves the MWI and wavefunction collapse. That’s a complicated issue I’d love to see you address at some point.
As an aside, user feedback, it took me a while to understand Figures 3. I think the little pyramid threw me (why a pyramid and not just a dot?), as well as the downward movement of the second case. While it’s not a spacetime diagram, the similarity to one had me expecting the collision to move upwards. But that’s just me. (I will mention that an extra “t” snuck into the Figure 3 caption.)
BTW, I’m enjoying your book!
Thanks for the comments. I may try to improve figures 3 and 4, I agree they are not ideally presented in the current text. Maybe there’s a way to help step the reader through from Figure 1 to Figure 3 more gently, but I haven’t found it yet.
As for wave function collapse vs multi-worlds; Yes. Before I do that, I should show some other examples. (It’s particularly important to show an example where two branches of a wave function can interfere and what the effects of that can be, but I haven’t thought of a simple one.)
Glad you are enjoying the book!
Your discussion related to Figure 5 above is a very good representation of what actually happens in Extended X-Ray Absorption Fine Structure (EXAFS) Spectroscopy, which is a useful tool for obtaining nearest-neighbor distances in non-crystalline samples for which X-ray diffraction doesn’t work. In EXAFS, a complex molecule (such as, say, a protein) is exposed to a spectrum of soft X-rays, and its absorption spectrum is measured as a function of photon energy. The absorption coefficient will be small at low energies, and will then rise abruptly when the X-ray energy corresponds to the ionization energy of an electron in some atom of the molecule (typically a transition metal). As the photon energy increases beyond this threshold, the absorption coefficient displays periodic “wiggles” which encode the distance from the atom being ionized to its nearest neighbors. This is because the electron in question seems to probe its immediate surroundings before “deciding” whether or not to leave its atom, with ionization being favored whenever the ionized electron can essentially stay in phase with itself if it reflects off of one or another of the atoms nearest to it — a real-world example of your third bullet point under Figure 5.
One more question:
(3) In all-particles wave function in your Final Thoughts, does a change such as two particles mutually annihilating propagate “instantly” (in the EPR sense) or at lightspeed? If the latter, doesn’t that propagation convert your wave functions into multi-particle wavicles?
See https://profmattstrassler.com/articles-and-posts/particle-physics-basics/fields-and-their-particles-with-math/8-how-particles-and-fields-interact/ . It is not instantaneous. In any case, the wave function of the system (which describes all the fields involved) specifically describes the transition from one set of wavicles into another set.
Hi Matt Strassler! A fun read, thank you. Two questions:
(1) Doesn’t QED treat photon exchanges as interactions that lack wave-like behavior?
(2) I live on Pluto. One day, a NASA probe propelled to high velocity by a solar sail strikes me and breaks my arm. Was I injured by waves or wavicles?
(1) No. Interactions don’t have behavior, in any case. QED describes interactions among wavelike objects. See https://profmattstrassler.com/articles-and-posts/particle-physics-basics/fields-and-their-particles-with-math/8-how-particles-and-fields-interact/
(2) You were injured by wavicles.