© Matt Strassler [September 1, 2012]
This is article 5 in the sequence entitled Fields and Particles: with Math. Here is the previous article.
Reminder: The Quantum Ball on a Spring
Back in the first article in this series we studied the ball (of mass M) on a spring (of strength K), and recalled that its oscillations have
- a motion formula: z(t) = z0 + A cos [ 2 π ν t ]
- energy: E = 2 π2 ν2 A2 M
- an equation of motion: d2z/dt2 = – K/M (z – z0)
where the equation of motion forces ν = √ K/M / 2π, but allows the amplitude A to be of any positive size. Then in the second article, we saw that what quantum mechanics does to the oscillations (among many more subtle things) is effectively restrict the amplitude — it can’t be just anything. Instead the amplitude is quantized; it has to take one of an infinite number of discrete values.
- A = (1/2 π) √ 2 n h / ν M
where n=0, or 1, or 2, or 3, or 44, or any integer greater than or equal to zero. In particular, A can be as small as (1/2 π) √ 2 h / ν M , but it can’t be anything smaller, except zero. We say that n is the number of quanta of oscillation in the ball’s motion. The energy of the ball is also now quantized:
- E = (n+1/2) h ν
The most important fact, for us, is that the energy required to add one quantum of oscillation to the ball’s motion is h ν; we may say that each quantum carries energy hν.
The Quantum Wave
For waves, it’s basically the same; we have, for a wave of frequency ν and wavelength λ oscillating with amplitude A around an equilibrium position Z0,
- a motion formula: Z(x,t) = Z0 + A cos (2π [ν t – x/λ])
- the energy per wavelength: 2 π2 ν2 A2 Jλ
(where Jλ is a constant that depends only on, say, the rope, if these are waves on a rope), and several possible equations of motion, of which we chose two to study
- Class 0: d2Z/dt2 – cw2 d2Z/dx2 = 0
- Class 1: d2Z/dt2 – cw2 d2Z/dx2 = – (2 π μ)2 (Z-Z0)
Again quantum mechanics restricts the amplitude A, which we might have thought could be of any size we liked, to discrete values. Just as for an oscillation of a spring,
- a (simple) wave of a given frequency and wavelength is made from n quanta
- the allowed values of the amplitude A are proportional to √ n ;
- the allowed values of the energy E are proportional to (n+1/2)
More precisely, just as for a ball on a spring
- the allowed values of the energy are E = (n+1/2) h ν
- each quantum of a wave carries energy h ν
The formula for A is a bit more complicated, because here we have to know how long the wave is, and an exact formula would be messy, so let me just write one that gets the right idea. We got most of our formulas studying waves that are infinite, but any real wave in nature has a finite length. If the wave is roughly of length L, and therefore has L/λ crests, then the amplitude is approximately
- A = (1/2 π) √ 2 n h λ / ν L Jλ
which is proportional to √ n h / ν just as for the spring, but depends on L; a longer wave has a smaller amplitude, arranged just such that each quantum of the wave always has energy h ν.
And that’s it, as illustrated in the figure below (which you may wish to compare with Figures 1 and 3 in the article on the Quantum Ball on a Spring.)
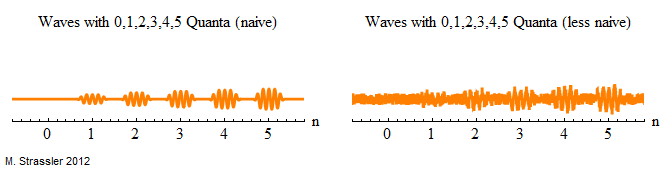
One Implication
What does this mean for our Class 0 and Class 1 waves?
Since waves that satisfy an equation of Class 0 can have any frequency, they can correspondingly have any energy. Even with a tiny amount of energy ε, you can always make a single quantum of a Class 0 wave with frequency ν = ε/h. For such small energy, that quantum wave will have very low frequency and very long wavelength, but it can exist.
Waves that satisfy an equation of Class 1 are different. Since there is a minimum frequency νmin = μ that such waves can have, there is a quantum of lowest energy
- Emin = h νmin = h μ
If your tiny amount of energy ε is less than this, you cannot make a quantum of this sort of wave. Quanta of Class 1 waves with finite wavelength and larger frequency all have E ≥ h μ .
To Sum Up
Before we account for quantum mechanics, the amplitude of a wave, just like the amplitude of a ball on a spring, can vary continuously; you can make it as large or as small as you want. But quantum mechanics implies there’s a smallest possible non-zero amplitude for a wave, just as for an oscillation of a ball on a spring; and in general only discrete values of the amplitude are allowed. The allowed amplitudes are such that for both an oscillating ball on a spring and a wave of any class with a definite frequency ν
- to add a single quantum of oscillation requires energy h ν
- with n quanta of oscillation, the energy of oscillation is (n+1/2) h ν
Now it’s time to apply this knowledge to fields, and observe when and how the quanta of the waves in these fields could be interpreted as what we call the “particles” of nature.


27 Responses
hey i have a question what happens if a trough is smaller than a crest on a any electromagnetic wave.
Professor Strassler ive been looking for a constantly updating and exeedingly current view on theoretical physics (something i pursue with pure tenacity) and ive found it here. thank you, i can further improve my understanding of these sciences.
Prof Strassler,
What is the equation that must be solved to determine the energy of a wave (ripple) in a field?
And, what are the constraints or boundary conditions on this equation such that its solution forces the possible values of the energy to be quantized?
Is “matter wave” transverse or longitudinal wave? or neither (its amplitude has no direction?)?
Be interesting to see how you merge into theoretic.:)
A simple string conversion perhaps?
Best,
Try this for printing and paste within html language at end of post.
This was a quick scan so there maybe others- http://www.printfriendly.com/button
Thanks — will try that.
Print: try the following, go to the top of the page and click on the right side of the mouse and scroll to “print” Cannot control whether this works because my printer needs to be replaced.
Very interesting. Thank you.
Thanks for the great series of articles, and thanks for not shying away from the mathematics either. Looking forward to the next one!
* the energy required to add one quantum of oscillation to the quantum ball’s motion is hv.
* to add a single quantum of oscillation to the quantum wave also requires energy hv.
My question is: do the quantum ball and the quantum wave have the same inertia?
Even two quantum balls with the same frequency v, and therefore the same energy per quantum, need not have the same mass and momentum. If I double the mass M of the ball and double the strength of the spring (the spring constant K), the energy per quantum remains the same. So the answer to your question (“do a quantum ball and a quantum wave that have the same frequency v have the same inertia”) is no.
Hello Professor Strassler. I may be humping in here a few years late, but I needed to point out that you continue to refer to the spring constant, K, as the strength. Technically it is actually the “stiffness of the spring”; ten force required to produce a unit extension
I also tried to print and it doesn’t work properly. I would love to have the wave articles on paper right in front of me. I’m just a layperson who is fascinated by particle physics but my math skills are poor. Will need to chew through those equations a few more times. Your articles, Professor Strassler, have all been very valuable and helpful for me in order to understand how nature works. Thank you very much.
good article, but not able to print the pages either to a printer or pdf file without truncating part of the page.
There are all kinds of button on this page but no print button.
Most (if not all) of other physics blogs have a “print” button or the page can be printed properly.
Hope this can be fixed.
Really not sure about the cause here or even where to look for a solution. Any readers with suggestions?
Hi Matt, could you please help me clarify some confusion that I have in my mind? How do you “picture” the quanta of a wave? When you say quanta I immediately think of photons (quantum of light), and I imagine them as something separated by space and time. I can understand the properties of one quanta of wave, i.e. it has the energy h ν and increases the amplitude (according to the formula you mentioned), but I cannot quite “picture” it.
I understand your question: let me think about whether to insert the answer into the current article, or into the later one on particles. I should have foreseen it.
I am trying to make a comparison between the quantum ball and a photon on the one side and between the quantum wave and a light wave on the other side.
That not (as stated) the right comparison to make. The right one is that
* a quantum of oscillation for the quantum ball is to the oscillation of a classical ball
as
* a photon (a quantum of oscillation) for the quantum electric/magnetic field is to the light wave of the classical electric/magnetic field.
We’ll get to this in a few days. Meanwhile, I still don’t see where inertia is hiding in your thinking.
Light seems to have inertia.
Light has energy and it has momentum, though I haven’t explained that yet. Still trying to understand the source and nature of your question.
Both the quantum ball and the quantum wave have the same inertia?
Hmmm. Where did “inertia” come in to the discussion? Could you clarify the source of your question?