If a quantum object, such as a proton, is made from wavicles, how can we make sense of and measure its size and shape? Here are two classic ways to do it.
- Scatter a diffuse beam of particles off the object, hard enough to see them bounce off, but gently enough so that the internal structure of the object, if any, plays no role.
- Strike the object hard enough that its internal structure jiggles, like the sloshing of milk in a container, or like the rattling of the contents of a box of chocolates.
The second approach is the method described in the book’s Chapter 17. But the first method requires less energy, has a longer history, and is the one that was initially used to show that the proton has a size.
The Method of Scattering
In a certain sense, the method of scattering is familiar. To learn the size of, say, a metal ball, we would naturally be inclined to shine a flashlight on it. The flashlight consists of a beam of subatomic particles — photons, the particles of light. The metal ball will reflect those photons that strike it. As a result, the light gives us two clues about the size of the object.
- The ball leaves a shadow on the wall behind it, whose size tells us the size of the ball.
- The ball scatters light in all backward directions, and from the pattern of this reflected light, we can infer the size and reflectivity of the ball.

For an object that is subatomic, the shadow is useless; the shadow’s size is ultra-microscopic, and we can’t measure it. But even from a subatomic object, the reflected light can be measured, and used to learn something about the object’s size.
The size of the nucleus was measured in just this way, as described in the book’s Chapter 6.3. The only differences from Fig. 1 are that the target was an atomic nucleus, and the nuclei of helium atoms were used in the beam in place of photons.
The proton’s size, too, was measured in this way, using an electron beam impinging on hydrogen gas. (Hydrogen gas consists only of electrons and protons, and the electrons in the hydrogen have a lesser impact on the electrons in the beam than do the protons.)
But there’s a complication. Because a proton has electric charge, it would exerts an electric attraction on an nearby electron — and so, it would deflect all electrons — even if it had no size. This electrical effect has to be accounted for. Fortunately, even though the scattering of electrons off of an electrically charged sphere is never absent, it changes with the sphere’s diameter, and so a careful measurement can reveal the sphere’s size.
This scattering experiment was done in pioneering research by McAllister and Hoftstadter. (Robert Hofstadter, who won the Nobel Prize for this work in 1961, was the father of mathematician and writer Douglas Hofstadter, author of “Gödel, Escher, Bach”.) Their studies showed that the scattering of electrons off of protons is inconsistent with a proton being a “point”, and most easily explained by the proton having a diameter about one million billionth of a meter — roughly half the diameter of a typical helium nucleus.

The Method of Excited States
Now here’s another approach, which is the one described in and around Figure 40 of Chapter 17. Again, the basic idea is simple. If I shake a box with stuff inside it, I can hear and feel the rattling of whatever makes up its interior. That rattling tells me there’s something inside.
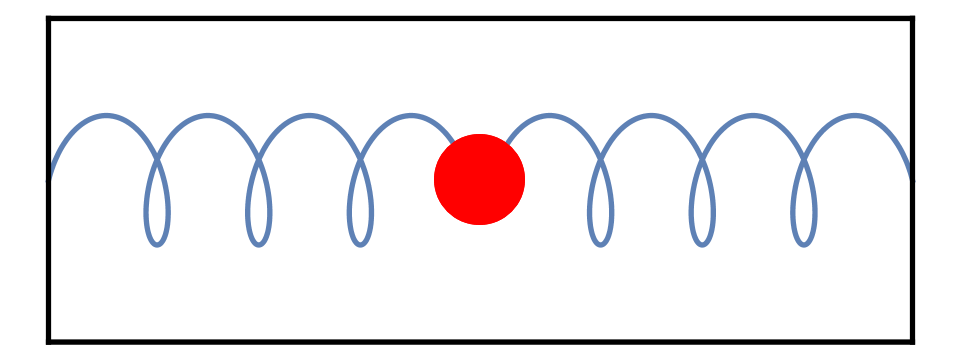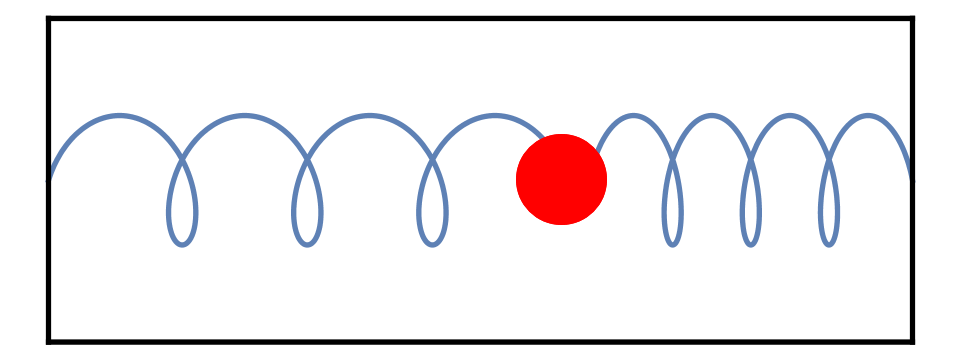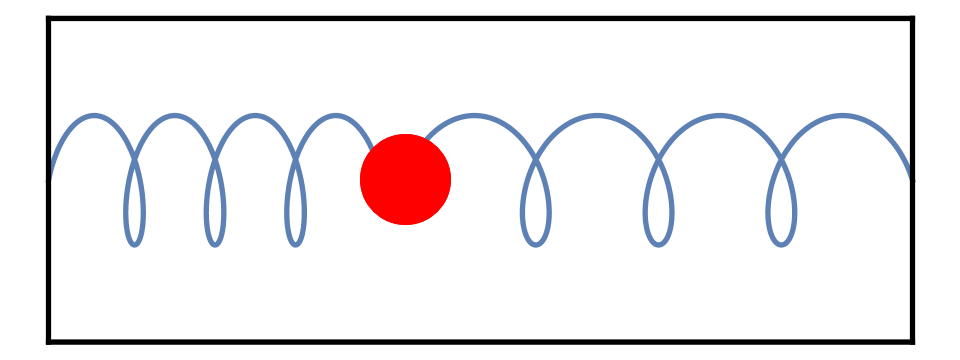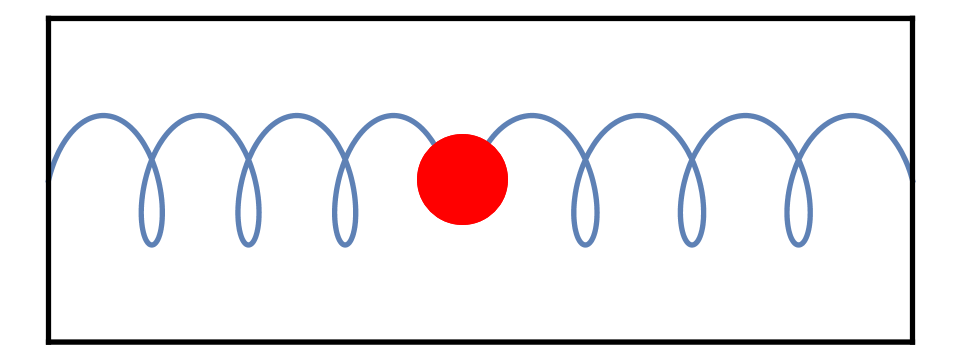
In daily life, the rattling usually stops as soon as I stop shaking the box. But that’s just because of friction between its contents. If instead I had a box with a single spring inside it, with low friction, then shaking the box would cause the springs to vibrate for quite a while, as in Fig. 3.
If it weren’t for quantum physics, any amount of shaking would be enough to make the spring vibrate. But a quantum box is different. The quantum formula E=f[h] states that if the spring has a resonant frequency f, then the minimal amount of energy needed to make it vibrate is f[h]. More generally, to make any quantum system vibrate internally — to “excite” the system — requires a minimum amount of energy.
Atoms
Any system with complex internal structure can vibrate in a whole variety of ways, and in a quantum system, each one requires a specific amount of energy. Atoms are examples; they are made of electrons and an atomic nucleus. In atoms, the various modes of vibration of their interiors are called “excited states”, and the amounts of energy needed to excite them are called the atom’s “energy levels.”
For any particular type of atom, there is a miminal amount of energy that it may have. This occurs when it is stationary and is in its “ground state”, with the least possible amount of internal motion. (This minimal energy, divided by [c2], is quoted as the atom’s rest mass.) The atom also has a huge number of “excited” states, in which the atom remains stationary but its internal structure rattles around in one way or another. Each particular excited state has a fixed amount of energy above the ground state (and thus slightly more rest mass).
A quantum object could in principle remain in an excited state forever, if it weren’t for dissipation. But dissipation is always present. Still, as noted in Chapter 21.2, dissipation in quantum physics is more suddent and dramatic than the slow, gradual effects of friction that we observe so often in daily life. The effect of dissipation is to cause what scientists call “atomic decay”, a sudden transition.
In an atomic decay, an atom in an excited state may suddenly return to its ground state, dissipating its excess energy by emitting a photon that carries that energy away. In this case, the photon will carry off exactly the difference between the energy of the excited state and the energy of the ground state; and measuring the energies of those photons gives insight into the excited states of the atom.
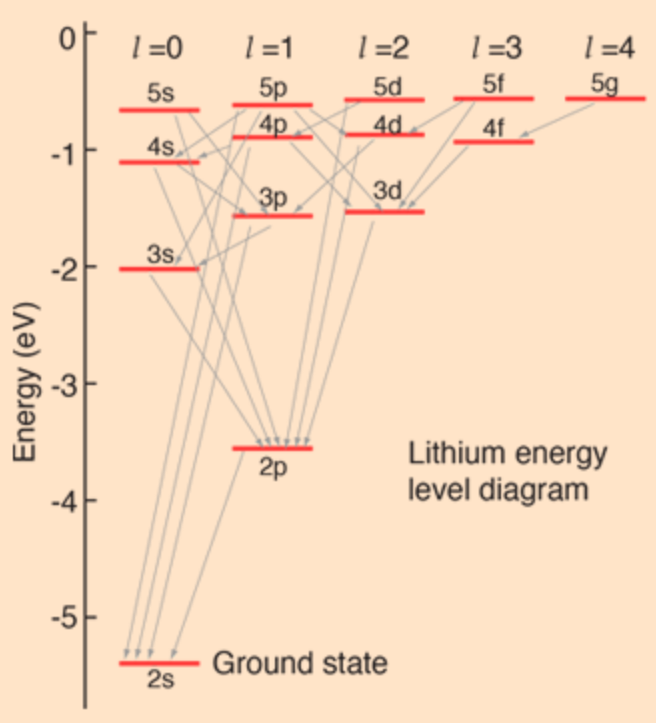
Or a decay may involve multiple steps, in which one excited state transitions to another, emitting one photon, and the second excited state returns to the ground state, emitting a second photon. (Such transitions in lithium are indicated by the arrows in Fig. 4, which show how the higher excited states can take many paths back down to the ground state.)
In each case, the photons emitted carry an amount of energy that is the difference between one state’s energy and another. By observing these photons, scientists can gain clues about the excited states of an atom, and from this information they can infer its internal structure. Indeed, the photons emitted by hydrogen were used by Niels Bohr in his initial model of the hydrogen atom.

Later, the photons emitted by more complex atoms were used to verify the more accurate models of atoms that emerged from quantum physics of the 1920s and 1930s. (Click here for more examples of the photons emitted in atomic transitions; note how different they can be from one type of atom to another.)
Protons
What works for atoms made from electrons and atomic nuclei works also for the nuclei themselves, and even for the protons and neutrons made from quarks. In particular, hard collisions of other particles with protons and neutrons reveal they have excited states, thus proving that they have internal structure.
An example of such a collision between an electron and a proton is shown in Fig. 6. The collision pops the proton into an excited state, called the “Delta” for historical reasons. This excited state rapidly decays back to a proton (or neutron) plus a particle called a “pion”, made also from quarks, anti-quarks and gluons, (but with no excess quarks over gluons.) [These pions in turn decay, but we need not go into those details.]
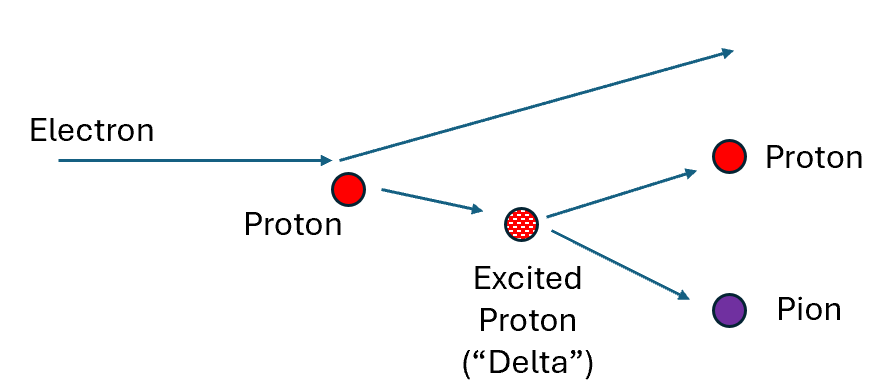
Other excited states of a proton can decay to a proton and more than one pion. This is the reasoning behind the book’s Figure 40, where at far right it is sketched how a proton, struck hard enough, is shrunk down small and raised to an excited state which quickly decays to two or more particles. This transition from one particle to many, via an excited state, is evidence that the proton has internal structure, like an atom. It confirms that a proton is not an elementary particle.

Estimating the Object’s Size From Its Excited States
[Added April 30, 2024, after a reader pointed out I hadn’t really addressed this properly. It is more technical than the rest of the article. Note that I composed this rather quickly, so there might some small errors hidden here; please point them out if you see them.]
Once we know that that an object has excited states, how can we estimate its size? To do so precisely, one has to look at details, making careful guesses about the system’s internals and shape based on what one learns about the excited states — specifically their energies and the patterns seen in their decays.
But one can already gain a rough measure of its size using some basic reasoning, the Heisenberg uncertainty principle of quantum physics, and dimensional analysis.
Some Rough Reasoning
The reasoning goes as follows. Suppose the object has mass M and length L. Suppose there is a piece of the object (let’s call it the “subobject“) that can vibrate within the object. Then we can use the uncertainty principle to estimate how much energy this vibration will have.
Even in the ground state, the subobject has some energy (this is zero-point energy). We can estimate it first. This being quantum physics, the subobject can spread out within the box, and can do so away from the box’s center by a distance of roughly L/2. By the uncertainty principle, that means the uncertainty in its momentum, even in the ground state, will be roughly (where
is called the “reduced Planck’s constant“).
Einstein’s relativity then tells us that the average energy of the vibrating subobject within the object will be given by its mass m, its average momentum-squared p2, and the cosmic speed limit c, by the following formula:
Now, when the object moves to the first excited state, the subobject vibrates a little more. But here’s the point: its typical momentum will still be proportional to , only somewhat larger. The logic of dimensional analysis can be used to show that there’s simply nothing else, in this situation, to which it could be proportional.
So let’s say the average momentum in the excited state is where
; that’s a pretty safe bet for an estimate. Then
The change in energy from the ground state to the first excited state,
can be written using an approximation for the difference of two square roots:
where in the last step I further assumed that , which lies between 0 and 3, is not so different from 2.
Now, solving this last equation for L gives
Since we’ve been making guesses about x, we can’t expect this to be more accurate than a factor of 2 or 3. But if we have no idea what the size is to start with, narrowing down the options to such a small factor is big progress.
Assumptions Needed
Now, this still isn’t quite enough to give us an estimate for L. The quantity is directly measurable, as the energy emitted in the decay from the 1st excited state to the ground state. But
is not directly measurable; it is the energy in the subobject within the ground state, which we don’t know. It might be a large fraction or a small fraction of the total energy of the full object.
The total energy of the full object is known, since by definition it is in terms of the mass of the full object. So now we have to make a guess; what fraction of
is
?
If the subobject is all of, or a substantial fraction of, the object, then we could take
in which case
Protons vs. Atoms
For a proton,
and
which is within a factor of 2 or so of the right answer.
For an atom, however, this method would give the wrong result, because inside of an atom the vibrating subobject is an electron, whose mass is much smaller than that of the atomic nucleus and of the atom as a whole. This wasn’t recognized until the famous experiments done in Rutherford’s lab by Geiger and Marsden, mentioned in Chapter 6.
Once armed with the knowledge that the mass of an atom sits in a small core, with the electrons on the outside and free to vibrate, one would guess that for an atom,
Since (recall a GeV is a billion eV), this gives
which again is more or less in the right ballpark.
A final note
Using rough-and-dirty methods like this is a bit of an art form — a mixture of intuition, approximation, educated guesses, and cross-checks against experimental data. When used in the wild by real physicists on unsolved problems, efforts are usually made to be more precise than I’ve been here. But even when more precision is not possible based on current information, the key steps forward are clear: first, to use these theoretical methods to make definite predictions for experiments to check, and second, to rely on experiments both to test those predictions and to provide additional information that can be used to refine the theoretical approach. With parallel advances in theory and in experiment, one can hope to come to a detailed and complete understanding of the object under study. That’s a process that may take decades, but it’s one with a fantastic success record.


19 Responses
Very nice article!
Great article, thank you!
I believe I did this in university or it’s a constructed memory, but a Scientific American article [which I ordinarily don’t read since it got too much popular science] about the strong force made me do the wavelength size estimate for hydrogen atom nucleus (proton) and shell (electron) from the de Broglie wavelength. [“The Secret to the Strongest Force in the Universe”, BY STANLEY J. BRODSKY, ALEXANDRE DEUR & CRAIG D. ROBERTS”, Sci Am, APRIL 16, 2024.]
Perhaps the right answer for the wrong reason, but the article claiming that QCD strength estimates can circumvent Landau poles and interestingly here “quarks are confined within nucleons, which means quark and gluon quantum loops cannot grow larger than the size of the proton.” Their reference refer to it as matter wavelengths, so that’s was what I was prompted to use. [Stanley J. Brodsky, Robert Shrock, Maximum wavelength of confined quarks and gluons and properties of quantum chromodynamics, Physics Letters B, Volume 666, Issue 1, 2008.] Naively I didn’t think the particle loop propagators were confined, but the results seem to imply otherwise and it seems a useful model.
By the way, there is a factor 2 in the extended text particle-in-a-box derivations that may be unnecessary!?
There could be — which 2 are you referring to?
The de Broglie wavelength is related to hc/L, so you can get the right answer that way — if I’m understanding what you’re suggesting. But also, keep in mind that Brodsky’s views on confinement are a little unconventional. Lattice calculations using computers are the state of the art.
There’s a nice macroscopic example of an object that stays in an excited state: the method of distinguishing if an egg is fresh or boiled.
The idea is to spin the egg on a table, and then touch it for an instant so it stops, and let go immediately. If the egg is boiled it will stay stopped. But if it’s fresh it will spin itself back up since the liquid inside is still moving.
Indeed! I’m not sure of the best analogue to this in quantum states; the internals of the egg and the eggshell have an unusual interaction which is not typical of composite systems in particle or atomic physics.
So, IF one can take the two-slit experiment as reference, will scattering “collapse” the wavefunction(s) in atoms and fundamental particles in general, and this is why we can only see (measure) the “excited states” are discrete levels and not as waves?
Correction: “this is why we can only see (measure) the “excited states” AS discrete levels and not as waves”
“as” , not “are”
Sorry
Waves do have discrete levels; that is why violin strings have harmonics (“overtones”) with specific frequencies.
So, if it is not like the two-slit experiment where the wavefunctions collapses, then is it corect to interpret the electron excites states are volumes (very tiny shells) where the electron energy pressure is at equilibrum to the nucleus energy pressure, equal and opposite, hence the scattering is caused by the denser shells?
Like using a stroboscope to see the harmonics on a vibrating string.
Prof. if this correct or any where close then there MUST be quantum gravity in play in the atom like gravity is in a black hole, but at different scales. And one way to formulated is to derive the wavefunctions with time (the time interval from one quantum state to another, same as in the energy-time uncertainty principle) going to zero at the center of both the atom and black hole. Yes/no?
Matt, I enjoyed your panel discussion at Berkeley the other day. Regarding the size/mass/excited states relation, here’s a related question. How do we know that the muon and tau aren’t just excited states of an electron with internal structure like atoms or protons? (Ditto of course for the heavier quarks.) If there were strongly bound constituents of high enough mass, the binding energy (due to a new force) could offset the constituent masses and the leptons would appear pointlike in scattering experiments well below the new energy scale. I think this might require some unnatural coincidence of unrelated large numbers (masses & binding energies) but I’m curious to hear your take. Maybe this is technicolor? It’s been a long time…
Great question. I’m writing a blog post to answer it; keep an eye out. Probably Friday or Monday.
Fussy note: the first few words of the April 30 addition have a doubled word: “Once we know that(1) that(2) an object has …”
I missed how the second method is related to the size of the particle under scrutiny… How are the excepted states induced by hard collisions tell us the size of the proton/neutron/electron?
You’re right, I really didn’t explain that. All I showed was that it has a size, but not how big it is… and of course, that’s a little complicated. Let me add something about it. Thanks for the feedback.
That would be great, thank you. By the way, as a physicist I agree with Sean Carroll on his praise of your book, you achieved something that no-one achieved before: no equations, but without compromises and making sure every statement is precisely correct… Wow, not easy. I am not a particle physicist but still you made me realise several misconceptions I came to believe about what Quantum Field Theory really means during my advanced graduate courses, in particular the Higgs field. At least I will not pass the same misconceptions to my kids or students now taking advanced physics courses… 😉
Thanks, it’s great to hear that you agree with Sean, since this was my guiding principle in writing the book. And I’m glad I clarified some things for you.
The section you requested has been added! It’s a little rough, and feel free to point out any issues that you see, but it shows how basic quantum physics can be used to go from energy measurements to a size estimate, if one makes an educated guess about the system’s interior. Of course, with many more measurements than just one excited state, one can start to be much more precise.
I just read it Matt, beautiful! The use of rough-and-dirty methods in physics has been the foundation of so many great ideas; you can understand a lot with this art. Thank you for the extra effort, I think this extra note clarified a lot, both about size determination of wavelets like protons but also about the thinking process of a particle physicist. 😉
Glad this was to your liking!